India on Wednesday ordered the closure of company behind killer cough syrups in Gambia
The government ordered Maiden Pharmaceutical Limited to stop manufacturing all drugs with immediate effect after the consumption of some drugs it manufactured killed many children in Gambia.
Health Minister of India’s northern state of Haryana, Anil Vij said the pharmaceutical company allegedly made defective cough syrups leading to the death of more than 60 children in the Gambia.
The minister said that as many as 12 flaws were found during a joint inspection conducted by federal and state drug departments at the pharmaceutical company.
“A notice has been served to the pharma company directing that total production shall be stopped,’’ Vij said.
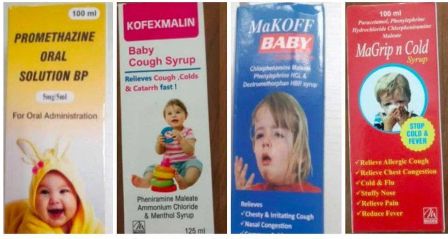
The pharma company had exported a variety of cough syrups to the Gambia where the deaths of infants were reported, and subsequently, the World Health Organization (WHO) issued product alerts about those cough syrups.
The WHO had linked four cough syrups, Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and MaGrip N Cold Syrup manufactured by the pharma company with the deaths in the Gambia.
“Samples of the drugs mentioned by the WHO of Sonipat’s pharmaceuticals company were sent to the Central Drug Lab in Kolkata. The reports are not in yet. Action will be taken after that,” Vij told the press in Haryana.
He said that the federal government was gathering complete information. (Xinhua/NAN)